Piperidinyl-1,2,3-triazole-urea-inhibitor-12
N1-substituted triazole urea previously disclosed as an inhibitor of ABHD6 KT195 was modified as N2-substituted isomer and shown to inhibit LYPLAL1. The compound 2 inhibited also other alpha/beta hydrolases and some SGNH hydrolase-type esterase (PAFAH1B2-3), but compound 11 and 12 are specific.
General
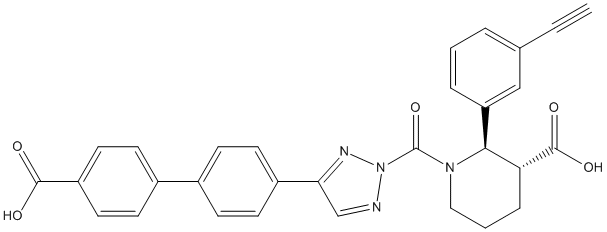
Type : Piperidine,Urea derivative,Triazol
Chemical_Nomenclature :
Canonical SMILES : C#CC1=CC(=CC=C1)[C@H]2[C@@H](CCCN2C(=O)[N]3N=CC(=N3)C4=CC=C(C=C4)C5=CC=C(C=C5)C(=O)O)C(=O)O
InChI : InChI=1S\/C30H24N4O5\/c1-2-19-5-3-6-24(17-19)27-25(29(37)38)7-4-16-33(27)30(39)34-31-18-26(32-34)22-12-8-20(9-13-22)21-10-14-23(15-11-21)28(35)36\/h1,3,5-6,8-15,17-18,25,27H,4,7,16H2,(H,35,36)(H,37,38)\/t25-,27+\/m1\/s1
InChIKey : PEXQGQZSCKJWJV-VPUSJEBWSA-N
Other name(s) :
MW : 520.54
Formula : C30H24N4O5
CAS_number :
PubChem :
UniChem : PEXQGQZSCKJWJV-VPUSJEBWSA-N
IUPHAR :
Wikipedia :
Target
Families : Piperidinyl-1,2,3-triazole-urea-inhibitor-12 ligand of proteins in family: LYsophospholipase_carboxylesterase
Stucture :
Protein : human-LYPLAL1
References (1)
| Title : Discovery of a Selective Covalent Inhibitor of Lysophospholipase-like 1 (LYPLAL1) as a Tool to Evaluate the Role of this Serine Hydrolase in Metabolism - Ahn_2016_ACS.Chem.Biol_11_2529 |
| Author(s) : Ahn K , Boehm M , Brown MF , Calloway J , Che Y , Chen J , Fennell KF , Geoghegan KF , Gilbert AM , Gutierrez JA , Kalgutkar AS , Lanba A , Limberakis C , Magee TV , O'Doherty I , Oliver R , Pabst B , Pandit J , Parris K , Pfefferkorn JA , Rolph TP , Patel R , Schuff B , Shanmugasundaram V , Starr JT , Varghese AH , Vera NB , Vernochet C , Yan J |
| Ref : ACS Chemical Biology , 11 :2529 , 2016 |
| Abstract : Ahn_2016_ACS.Chem.Biol_11_2529 |
| ESTHER : Ahn_2016_ACS.Chem.Biol_11_2529 |
| PubMedSearch : Ahn_2016_ACS.Chem.Biol_11_2529 |
| PubMedID: 27391855 |
| Gene_locus related to this paper: human-LYPLAL1 |