PNPPC
not to be confounded with para-nitrophenylphosphorylcholine (pNPPC)
General
Type : pNP,Carbamate
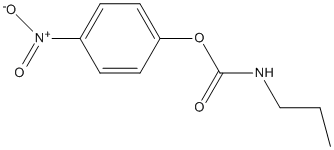
Chemical_Nomenclature : (4-nitrophenyl) N-propylcarbamate
Canonical SMILES : CCCNC(=O)OC1=CC=C(C=C1)[N+](=O)[O-]
InChI : InChI=1S\/C10H12N2O4\/c1-2-7-11-10(13)16-9-5-3-8(4-6-9)12(14)15\/h3-6H,2,7H2,1H3,(H,11,13)
InChIKey : FTPSVHHZKHVFNB-UHFFFAOYSA-N
Other name(s) : p-nitrophenyl-N-propyl carbamate,Carbamic acid, propyl-, 4-nitrophenyl ester,SCHEMBL14617269,CTK1I7375,Propylcarbamic acid 4-nitrophenyl ester,OR311785,(4-nitrophenyl) N-propylcarbamate,4-Nitrophenyl propylcarbamate,DTXSID50559139
MW : 224.21
Formula : C10H12N2O4
CAS_number : 63321-49-3
PubChem : 14343338
UniChem : FTPSVHHZKHVFNB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (1)
| Title : Human acylpeptide hydrolase. Studies on its thiol groups and mechanism of action - Scaloni_1994_J.Biol.Chem_269_15076 |
| Author(s) : Scaloni A , Barra D , Jones WM , Manning JM |
| Ref : Journal of Biological Chemistry , 269 :15076 , 1994 |
| Abstract : Scaloni_1994_J.Biol.Chem_269_15076 |
| ESTHER : Scaloni_1994_J.Biol.Chem_269_15076 |
| PubMedSearch : Scaloni_1994_J.Biol.Chem_269_15076 |
| PubMedID: 8195144 |