N-acyl-prolylpyrrolidine-inhibitor-2
General
Type : Pyrrolidine,Triazol,Carbamate,tert-Butyloxycarbonyl,tert-Butyl
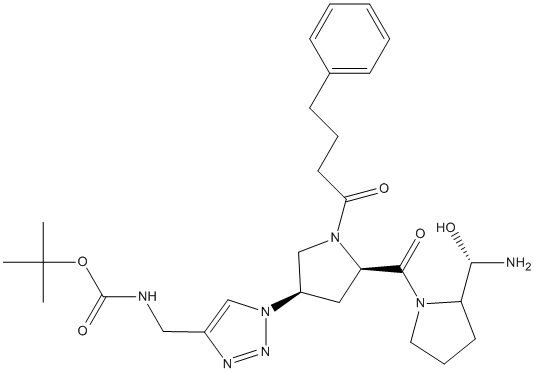
Chemical_Nomenclature : tert-butyl N-[[1-[(3S,5S)-5-[(2S)-2-[(R)-amino(hydroxy)methyl]pyrrolidine-1-carbonyl]-1-(4-phenylbutanoyl)pyrrolidin-3-yl]triazol-4-yl]methyl]carbamate
Canonical SMILES : CC(C)(C)OC(=O)NCC1=CN(N=N1)C2CC(N(C2)C(=O)CCCC3=CC=CC=C3)C(=O)N4CCCC4C(N)O
InChI : InChI=1S\/C28H41N7O5\/c1-28(2,3)40-27(39)30-16-20-17-35(32-31-20)21-15-23(26(38)33-14-8-12-22(33)25(29)37)34(18-21)24(36)13-7-11-19-9-5-4-6-10-19\/h4-6,9-10,17,21-23,25,37H,7-8,11-16,18,29H2,1-3H3,(H,30,39)\/t21-,22-,23-,25+\/m0\/s1
InChIKey : MVZCEONBTBQGTK-KELBGUDLSA-N
Other name(s) : JKT
MW : 555.68
Formula : C28H41N7O5
CAS_number :
PubChem : 70789753
UniChem : MVZCEONBTBQGTK-KELBGUDLSA-N
IUPHAR :
Wikipedia :
Target
Families : N-acyl-prolylpyrrolidine-inhibitor-2 ligand of proteins in family: S9N_PPCE_Peptidase_S9
Stucture : 4BCC Prolyl Oligopeptidase from porcine brain with a covalently bound P2-substituted N-acyl-prolylpyrrolidine inhibitor 2
Protein : pig-ppce
References (1)
| Title : P2-substituted N-acylprolylpyrrolidine inhibitors of prolyl oligopeptidase: biochemical evaluation, binding mode determination, and assessment in a cellular model of synucleinopathy - Van der Veken_2012_J.Med.Chem_55_9856 |
| Author(s) : Van der Veken P , Fulop V , Rea D , Gerard M , Van Elzen R , Joossens J , Cheng JD , Baekelandt V , De Meester I , Lambeir AM , Augustyns K |
| Ref : Journal of Medicinal Chemistry , 55 :9856 , 2012 |
| Abstract : Van der Veken_2012_J.Med.Chem_55_9856 |
| ESTHER : Van der Veken_2012_J.Med.Chem_55_9856 |
| PubMedSearch : Van der Veken_2012_J.Med.Chem_55_9856 |
| PubMedID: 23121075 |
| Gene_locus related to this paper: pig-ppce |