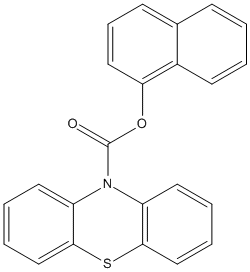
N-10-Phenothiazine-1-Naphtyl-carbamate
(Compound 28 Darvesh et al. 2008) Ki BCHE 0.036 micro M (for comparison Donepezil 2.21 for BCHE and 0.024 for ACHE), Ka ACHE 1650 M-1min-1 (for comparison rivastigmine 1130 M-1min-1)
General
Type : Phenothiazine,Carbamate,Sulfur Compound
Chemical_Nomenclature : naphthalen-1-yl phenothiazine-10-carboxylate
Canonical SMILES : C1=CC=C2C(=C1)C=CC=C2OC(=O)N3C4=CC=CC=C4SC5=CC=CC=C53
InChI : InChI=1S\/C23H15NO2S\/c25-23(26-20-13-7-9-16-8-1-2-10-17(16)20)24-18-11-3-5-14-21(18)27-22-15-6-4-12-19(22)24\/h1-15H
InChIKey : BJICBLMMTYZFKB-UHFFFAOYSA-N
Other name(s) : CHEMBL451838,naphthalen-1-yl 10H-phenothiazine-10-carboxylate,BDBM50292622,ZINC27529770,AKOS002279100
MW : 369.4
Formula : C23H15NO2S
CAS_number :
PubChem : 16645664
UniChem : BJICBLMMTYZFKB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : N-10-Phenothiazine-1-Naphtyl-carbamate ligand of proteins in family: BCHE
Stucture :
Protein : human-BCHE
References (2)
| Title : Selectivity of phenothiazine cholinesterase inhibitors for neurotransmitter systems - Darvesh_2013_Bioorg.Med.Chem.Lett_23_3822 |
| Author(s) : Darvesh S , Macdonald IR , Martin E |
| Ref : Bioorganic & Medicinal Chemistry Lett , 23 :3822 , 2013 |
| Abstract : Darvesh_2013_Bioorg.Med.Chem.Lett_23_3822 |
| ESTHER : Darvesh_2013_Bioorg.Med.Chem.Lett_23_3822 |
| PubMedSearch : Darvesh_2013_Bioorg.Med.Chem.Lett_23_3822 |
| PubMedID: 23707254 |
| Title : Carbamates with differential mechanism of inhibition toward acetylcholinesterase and butyrylcholinesterase - Darvesh_2008_J.Med.Chem_51_4200 |
| Author(s) : Darvesh S , Darvesh KV , McDonald RS , Mataija D , Walsh R , Mothana S , Lockridge O , Martin E |
| Ref : Journal of Medicinal Chemistry , 51 :4200 , 2008 |
| Abstract : Darvesh_2008_J.Med.Chem_51_4200 |
| ESTHER : Darvesh_2008_J.Med.Chem_51_4200 |
| PubMedSearch : Darvesh_2008_J.Med.Chem_51_4200 |
| PubMedID: 18570368 |