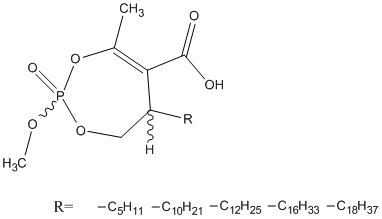
Cyclophostin-enolphosphonate
Enolphosphonate analogues of Cyclophostin. Fusarium solani cutinase was highly diastereoselective for the (Sp) configuration using (Sc) inhibitors, whereas no obvious stereopreference at phosphorus was observed with (Rc) compounds. Rv0183 from Mycobacterium tuberculosis exhibited strong enantioselective discrimination for (Sp) configuration regardless of the chirality at the asymmetric carbon atom. LipY from Mycobacterium tuberculosis discriminated only the unusual diastereoisomeric configuration (Rc, Rp) leading to the most potent inhibitor.
General
Type : Organophosphate,Cyclic OP,Lipase inhibitor
Chemical_Nomenclature :
Canonical SMILES :
InChI :
InChIKey :
Other name(s) :
MW :
Formula :
CAS_number :
PubChem :
UniChem :
IUPHAR :
Wikipedia :
Target
Families : Cyclophostin-enolphosphonate ligand of proteins in family: Cutinase || Monoglyceridelipase_lysophospholip
Stucture :
Protein :
References (1)
| Title : Enantioselective inhibition of microbial lipolytic enzymes by nonracemic monocyclic enolphosphonate analogues of cyclophostin - Point_2013_J.Med.Chem_56_4393 |
| Author(s) : Point V , Malla RK , Carriere F , Canaan S , Spilling CD , Cavalier JF |
| Ref : Journal of Medicinal Chemistry , 56 :4393 , 2013 |
| Abstract : Point_2013_J.Med.Chem_56_4393 |
| ESTHER : Point_2013_J.Med.Chem_56_4393 |
| PubMedSearch : Point_2013_J.Med.Chem_56_4393 |
| PubMedID: 23651298 |