CHEMBL4207101
deoxyvasicinone derivative Inhibition hAChE 7.61 +/- 0.53 nM, hBChE IC50 2.35 +/- 0.14 nM A1-42 self-aggregation (63.9 +/- 4.9%, 10 M), excellent metal chelator
General
Type : Derivative of Deoxyvasicinone,Quinazoline
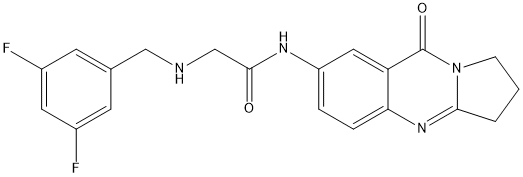
Chemical_Nomenclature : 2-[(3,5-difluorophenyl)methylamino]-N-(9-oxo-2,3-dihydro-1H-pyrrolo[2,1-b]quinazolin-7-yl)acetamide
Canonical SMILES : C1CC2=NC3=C(C=C(C=C3)NC(=O)CNCC4=CC(=CC(=C4)F)F)C(=O)N2C1
InChI : InChI=1S\/C20H18F2N4O2\/c21-13-6-12(7-14(22)8-13)10-23-11-19(27)24-15-3-4-17-16(9-15)20(28)26-5-1-2-18(26)25-17\/h3-4,6-9,23H,1-2,5,10-11H2,(H,24,27)
InChIKey : NLUCFDWSIZAJOW-UHFFFAOYSA-N
Other name(s) : BDBM50457222,Compound 12q
MW : 384.4
Formula : C20H18F2N4O2
CAS_number :
PubChem : 145977178
UniChem : NLUCFDWSIZAJOW-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : CHEMBL4207101 ligand of proteins in family: BCHE || ACHE
Stucture :
Protein : human-BCHE || human-ACHE
References (1)
| Title : Novel deoxyvasicinone derivatives as potent multitarget-directed ligands for the treatment of Alzheimer's disease: Design, synthesis, and biological evaluation - Ma_2017_Eur.J.Med.Chem_140_118 |
| Author(s) : Ma F , Du H |
| Ref : Eur Journal of Medicinal Chemistry , 140 :118 , 2017 |
| Abstract : Ma_2017_Eur.J.Med.Chem_140_118 |
| ESTHER : Ma_2017_Eur.J.Med.Chem_140_118 |
| PubMedSearch : Ma_2017_Eur.J.Med.Chem_140_118 |
| PubMedID: 28923380 |