CHEMBL1910114
similar to CHEMBL1910111 CHEMBL1909991 CHEMBL2441952
General
Type : Pyrrolidine,Pyridine
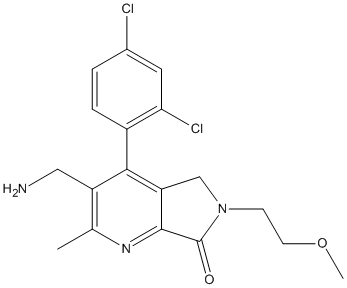
Chemical_Nomenclature : 3-(aminomethyl)-4-(2,4-dichlorophenyl)-6-(2-methoxyethyl)-2-methyl-5H-pyrrolo[3,4-b]pyridin-7-one
Canonical SMILES : CC1=C(C(=C2CN(C(=O)C2=N1)CCOC)C3=C(C=C(C=C3)Cl)Cl)CN
InChI : InChI=1S\/C18H19Cl2N3O2\/c1-10-13(8-21)16(12-4-3-11(19)7-15(12)20)14-9-23(5-6-25-2)18(24)17(14)22-10\/h3-4,7H,5-6,8-9,21H2,1-2H3
InChIKey : CLNAIMPDNPYUGV-UHFFFAOYSA-N
Other name(s) : SCHEMBL2704246,BDBM50356577,3-(Aminomethyl)-4-(2,4-Dichlorophenyl)-6-(2-Methoxyethyl)-2-Methyl-5,6-Dihydro-7h-Pyrrolo[3,4-B]pyridin-7-One,KXB
MW : 380.26
Formula : C18H19Cl2N3O2
CAS_number :
PubChem : 15950615
UniChem : CLNAIMPDNPYUGV-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : CHEMBL1910114 ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 3SWW Crystal structure of human DPP-IV in complex with SA-(+)-3-(aminomethyl)-4-(2,4-dichlorophenyl)-6-(2-methoxyethyl)-2-methyl-5H-pyrrolo[3,4-B]pyridin-7(6H)-one
Protein : human-DPP4
References (1)
| Title : 7-Oxopyrrolopyridine-derived DPP4 inhibitors-mitigation of CYP and hERG liabilities via introduction of polar functionalities in the active site - Wang_2011_Bioorg.Med.Chem.Lett_21_6646 |
| Author(s) : Wang W , Devasthale P , Wang A , Harrity T , Egan D , Morgan N , Cap M , Fura A , Klei HE , Kish K , Weigelt C , Sun L , Levesque P , Li YX , Zahler R , Kirby MS , Hamann LG |
| Ref : Bioorganic & Medicinal Chemistry Lett , 21 :6646 , 2011 |
| Abstract : Wang_2011_Bioorg.Med.Chem.Lett_21_6646 |
| ESTHER : Wang_2011_Bioorg.Med.Chem.Lett_21_6646 |
| PubMedSearch : Wang_2011_Bioorg.Med.Chem.Lett_21_6646 |
| PubMedID: 21996520 |
| Gene_locus related to this paper: human-DPP4 |