AI6-7QBR
General
Type : Quinoline,Piperazine,tert-Butyl,Multitarget,Monoamine-oxidase-inhibitor
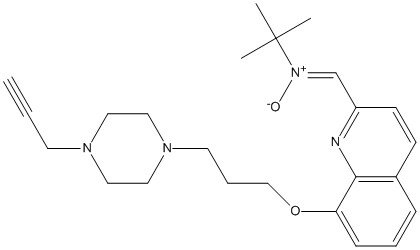
Chemical_Nomenclature : N-tert-butyl-1-[8-[3-(4-prop-2-ynylpiperazin-1-yl)propoxy]quinolin-2-yl]methanimine oxide
Canonical SMILES : CC(C)(C)\/[N+](=C\/c1ccc2cccc(c2n1)OCCCN3CCN(CC3)CC#C)\/[O-]
InChI : InChI=1S\/C24H32N4O2\/c1-5-12-26-14-16-27(17-15-26)13-7-18-30-22-9-6-8-20-10-11-21(25-23(20)22)19-28(29)24(2,3)4\/h1,6,8-11,19H,7,12-18H2,2-4H3\/b28-19-
InChIKey : JEJKPOMBHZMGMS-USHMODERSA-N
Other name(s) : (Z)-N-tert-butyl-1-(8-(3-(4-(prop-2-yn-1-yl)piperazin-1-yl)propoxy)quinolin-2-yl)methanimine oxide,~{N}-~{tert}-butyl-1-[8-[3-(4-prop-2-ynylpiperazin-1-yl)propoxy]quinolin-2-yl]methanimine oxide
MW : 408.54
Formula : C24H32N4O2
CAS_number :
PubChem :
UniChem : JEJKPOMBHZMGMS-USHMODERSA-N
IUPHAR :
Wikipedia :
References (1)
| Title : 8-Hydroxyquinolylnitrones as multifunctional ligands for the therapy of neurodegenerative diseases - Knez_2023_Acta.Pharmaceutica.Sinica.B_13_2152 |
| Author(s) : Knez D , Diez-Iriepa D , Chioua M , Gottinger A , Denic M , Chantegreil F , Nachon F , Brazzolotto X , Skrzypczak-Wiercioch A , Meden A , Pislar A , Kos J , Zakelj S , Stojan J , Salat K , Serrano J , Patricia Fernandez AP , Sanchez-Garcia A , Martinez-Murillo R , Binda C , Lopez-Munoz F , Gobec S , Marco-Contelle J |
| Ref : Acta Pharmaceutica Sinica B , 13 :2152 , 2023 |
| Abstract : Knez_2023_Acta.Pharmaceutica.Sinica.B_13_2152 |
| ESTHER : Knez_2023_Acta.Pharmaceutica.Sinica.B_13_2152 |
| PubMedSearch : Knez_2023_Acta.Pharmaceutica.Sinica.B_13_2152 |
| PubMedID: 37250172 |
| Gene_locus related to this paper: human-BCHE |